Newsletter Insights
Newsletter Signup
Signup to get access to our regular monthly EU Insights newsletter and stay up to date with all the latest from PAREA.
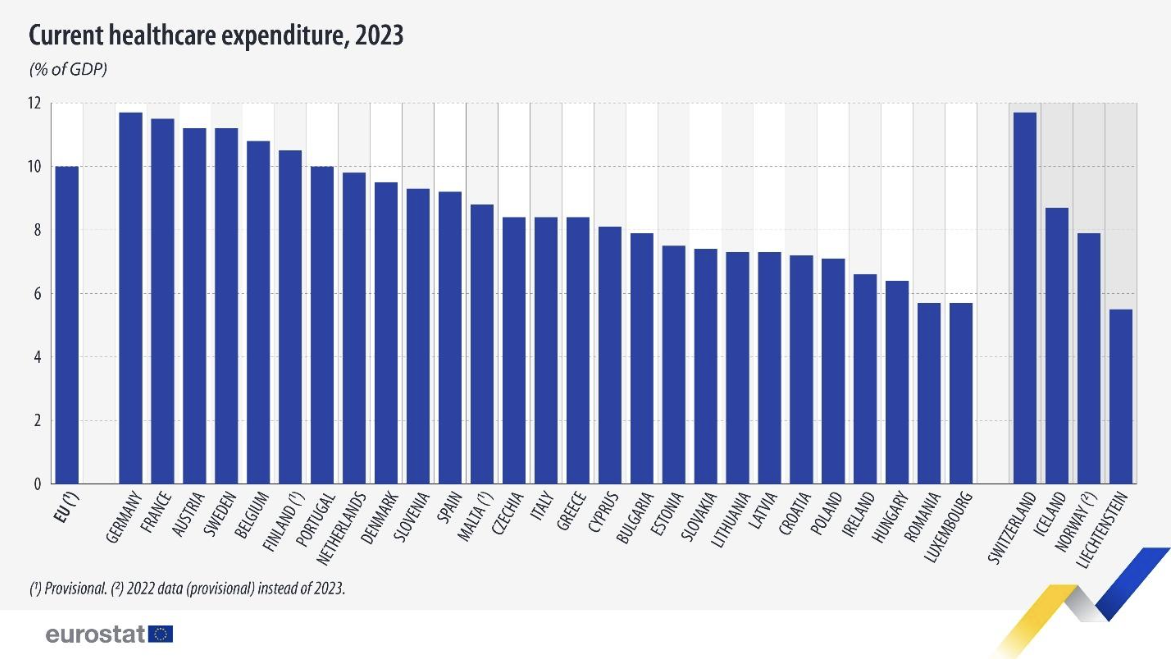
EU healthcare spending reaches 10% of GDP, amounting to €1.72 trillion in 2023
Healthcare expenditure in the European Union reached 10% of GDP in 2023, according to newly released Eurostat figures. This confirms healthcare as one of the largest areas of public and private spending across the EU, reflecting both post-pandemic pressures and longer-term trends linked to ageing populations, workforce shortages, and rising demand for care.
Biotech Act adopted: stronger incentives and faster trials to boost EU biotech
The European Union has formally adopted the Biotech Act, marking a significant step in its efforts to strengthen Europe’s position in global biotechnology. The legislation introduces longer periods of market exclusivity for medicines that are developed and manufactured in the EU, alongside commitments to speed up clinical trial procedures.
PAREA at the European Parliament launch of Headway Mental Health Index 5.0
On 10 December, Francisca Silva, Project Officer at PAREA, attended the European Parliament event launching the Headway Mental Health Index 5.0, developed by Angelini Pharma and TEHA Group | The European House – Ambrosetti (TEHA Group).
PAREA takes the stage at PSYCH Symposium 2025
On 4 December, Francisca Silva, Project Officer at PAREA, took part in PSYCH Symposium in London - this year co-organised with Drug Science. The symposium is one of Europe's leading gatherings for the industry’s most influential figures to connect, collaborate, and expedite the adoption of psychedelic healthcare.
Horizon Europe 2026–27: final work programmes promise broader, less prescriptive calls
The European Commission has adopted the final set of Horizon Europe work programmes for the years 2026–2027, outlining about €41 billion in funding for research and innovation projects across all clusters and missions. This marks the last programming cycle under Horizon Europe before the EU moves to its next framework programme, FP10.
Patient-centred care key to addressing unmet mental health needs
A new report by PAREA’s member GAMIAN-Europe, Patient-centred Solutions to Addressing Unmet, Complex and Multiple Mental Health Needs, reinforces that access to appropriate mental health care is a fundamental human right, yet many people across Europe continue to struggle to receive timely, coordinated, and effective support.
Introducing the Independent Psychedelic Evidence Assessment Working Group (IPEA-WG)
The Independent Psychedelic Evidence Assessment Working Group (IPEA-WG) has been launched at the Faculdade de Medicina da Universidade de Lisboa, with support from Norrsken Mind. This multidisciplinary group brings together scientists, philosophers and health experts to provide rigorous, independent guidance on the evaluation of psychedelic therapies.
EU research and development spending exceeded €403 billion in 2024
New Eurostat figures show that the European Union invested €403.1 billion in research and development (R&D) in 2024, a rise of 3.2% compared with 2023. This continues a steady upward trend over the past decade, with gross domestic expenditure on R&D increasing significantly in most Member States.
EU-funded project to build resilient and mentally healthy societies
REMESOS is a new, EU-funded programme coordinated by EuroHealthNet designed to strengthen mental health and social resilience across European countries. The project responds to persistent post-pandemic pressures and widening inequalities by combining population-level prevention and promotion with service-level transformation, creating an integrated approach that links public-health action with improvements in care delivery.
What Europe’s health systems will require to implement psychedelic therapies
A new article published in European Neuropsychopharmacology provides a critical assessment of what psychedelic therapies would need in order to be integrated into European healthcare systems. The authors stress that Health Technology Assessment (HTA) bodies across Europe will ultimately determine whether these therapies are reimbursed and accessible.
Australia becomes first to reimburse psychedelic therapy for veterans
The Australian Government has taken a historic step by approving federal funding for psychedelic-assisted psychotherapy (PAP) for veterans suffering from post-traumatic stress disorder (PTSD) and treatment-resistant depression (TRD). Through the Department of Veterans’ Affairs (DVA), eligible veterans will now be able to access MDMA or psilocybin treatments within a regulated clinical setting at no personal cost.
Paving the Way for Implementing Psychedelic Therapies in Europe
PAREA unites pan-European patient organizations, medical associations, scientific societies, and psychedelic foundations to promote evidence-based policymaking and prepare health systems for the adoption of psychedelic therapies.
PAREA Supports the Psychedelic Lived Experiences Summit
PAREA is a supporting partner of the Psychedelic Lived Experiences Summit, taking place November 21–23. This free, online event focuses on the realities of psychedelic care by placing lived experience at the center of the conversation.
€5 billion Scaleup Europe Fund aims to close the EU’s investment gap in high-growth innovation
The European Investment Bank (EIB) and European Investment Fund (EIF) have secured commitments from a group of leading institutional investors to create the Scaleup Europe Fund, a €5 billion initiative to help high-growth European companies stay and grow within the EU.
Help advance psychedelic research: PsyPal trial of psilocybin therapy in palliative care is recruiting across Europe
Psilocybin Therapy for Psychological Distress in Palliative Care Patients (PsyPal) - the first EU-funded clinical trial of psychedelic therapy - is now underway and actively recruiting participants across its four trial sites in Copenhagen, Groningen, Lisbon and Prague.
PAREA takes the stage at IMBAS Psychedelic Research Conference, at Trinity College Dublin
On October 31 and November 1, PAREA participated in the IMBAS Psychedelic Research Conference at Trinity College Dublin, the second edition of Ireland's leading event on psychedelic science.
PAREA at LaPsyConf 2025: shaping the future of mental health in Latin America
In October, PAREA participated in LaPsyConf 2025, the first international conference in Buenos Aires dedicated to rethinking mental health, longevity, and emerging therapeutics. Tadeusz Hawrot, Founder of PAREA, delivered the opening address titled “Global Issues: Mental Health Indicators, Global Regulation, and Economic Impact.”
Spain adopts first national regulation for medical cannabis use
Spain’s Council of Ministers has formally approved a Royal Decree establishing the country’s first regulatory framework for the medical use of cannabis in standardised preparations. The decision aims to provide an alternative therapeutic option when conventional treatments fail.